David S. Saperstein, Todd D. Levine, Madison Levine, and Nicole Hank
Phoenix Neurological Associates, Phoenix, Arizona, USA
ABSTRACT
Objective: To assess the usefulness of skin biopsy in the assessment of patients with suspected small fiber neuropathy (SFN).
Methods: Retrospective chart review of patients with sensory symptoms or findings restricted to small nerve fibers and normal nerve conduction studies (NCS) seen in a subspecialty neuromuscular private practice.
Results: Assessments were made on 145 patients. Skin biopsy was abnormal in at least one site in 86 patients (59%). There was no significant difference between patients with normal or abnormal skin biopsies with respect to age, gender, or duration of symptoms. Compared to patients with normal skin biopsies, patients with confirmed SFN were significantly more likely to have pain and were more than twice as likely to respond to standard neuropathic pain medications.
Conclusions: Skin biopsy is useful in the diagnosis and management of patients with otherwise unexplained sensory symptoms or findings.
INTRODUCTION
Neuropathic pain is a symptom commonly encountered by neurologists. While this is commonly caused by peripheral neuropathy, a number of these patients will have no demonstrable evidence for peripheral nerve abnormalities on nerve conduction studies (NCS) or even on clinical exam [1]. Such patients may have a small fiber neuropathy (SFN), but additional considerations include central nervous system disorders, non-neurological disease, or even a nonorganic process. SFN can produce various patterns of involvement. Most patients present with typical, symmetrical, distal, and stocking/glove symptoms [2]. However, some patients will have much more proximal and/or multifocal involvement than would be expected with typical peripheral neuropathies, suggestive of ganglionopathies [3–5]. Most patients with SFN will have abnormal pinprick and light touch sensation, but more than a third will have a normal sensory examination [1]. Some patients may have autonomic symptoms such as orthostasis, impotence, bladder symptoms, bowel abnormalities, and sweating abnormalities [1, 2, 6]. Immunohistochemical analysis of intraepidermal nerve fiber density (IENFD) from a skin biopsy can be a useful tool to identify SFN [7, 8]. In the last few years, commercially available testing for SFN has allowed clinicians, without access to academic centers, to easily measure IENFD. A reduction in the density of these small fibers has become a gold standard for the diagnosis of SFN [8]. Obtaining biopsies from both a distal and proximal site in an affected limb can indicate whether or not a SFN is length dependent, and this has become the standard approach to SFN testing with skin biopsy [7, 8]. Patients with SFN that is nonlength dependent are more likely to have diabetes, impaired glucose tolerance, or an immune-mediated process as the cause for the neuropathy [3–5]. Although somewhat invasive, this test is typically more accessible to clinicians than other tests for SFN, such as quantitative sensory testing (QST) and autonomic testing. Skin biopsy is much less invasive and more practical than cutaneous nerve biopsy. While skin biopsy can indicate that a patient’s symptoms represent SFN, questions remain as to how useful this information is and whether the results of skin biopsy can alter patient management. However, many clinicians are reluctant to perform these biopsies because of the belief that biopsy will not add helpful clinical information. To explore these questions, we reviewed clinical and laboratory data from patients in our practice who had undergone skin biopsy testing to evaluate sensory symptoms.
METHODS
We conducted a retrospective chart review from a subspecialty neuromuscular private practice. All patients between January 2005 and June 2008 who underwent a skin biopsy for suspected SFN were included. The patients had sensory symptoms of numbness or dysesthetic sensations such as burning or stinging. Symptoms could be length dependent or multifocal. Objective sensory deficits were not required but patients could not have exam findings indicating large fiber involvement: abnormal deep tendon reflexes, vibration sensation, or proprioception. Patients with abnormal NCS were also excluded. All patients underwent a standardized battery of laboratory blood tests: complete blood count, electrolytes, creatinine, blood urea nitrogen, liver function tests, fasting glucose, 2-hr oral glucose tolerance test, ANA, ESR, thyroid function tests, vitamin B12, methylmalonic acid, homocysteine, and serum immune fixation electrophoresis. All of the biopsies were performed in our office and were evaluated in a blinded manner by Therapath (New York, USA). Specimens were processed and IENFD determined using methods previously described [7]. In most patients, two biopsies were obtained from standard sites: distal calf and proximal thigh [7]. When available, age- and gender-matched normal values were used to determine whether or not IENFD was normal. Such normal values are available only for the distal calf [9]. For other sites, we used normal values established by the reference lab performing the testing (which were similar to those published in the literature) [7]. Collected information about clinical symptoms included the location at onset, the anatomical distribution, and the characteristics of the neuropathic symptoms. Medical history and laboratory test results were reviewed to look for potential causes of the neuropathic complaints. In addition, we evaluated which medications the patients had been treated with and whether there was a positive response to the medication. A patient was considered to have a positive response to a symptomatic medication if they experienced at least a 30% decrease in pain and they remained on therapy for greater than 3 months. The data was analyzed using the paired t-test for continuous variables and Chi square for proportions.
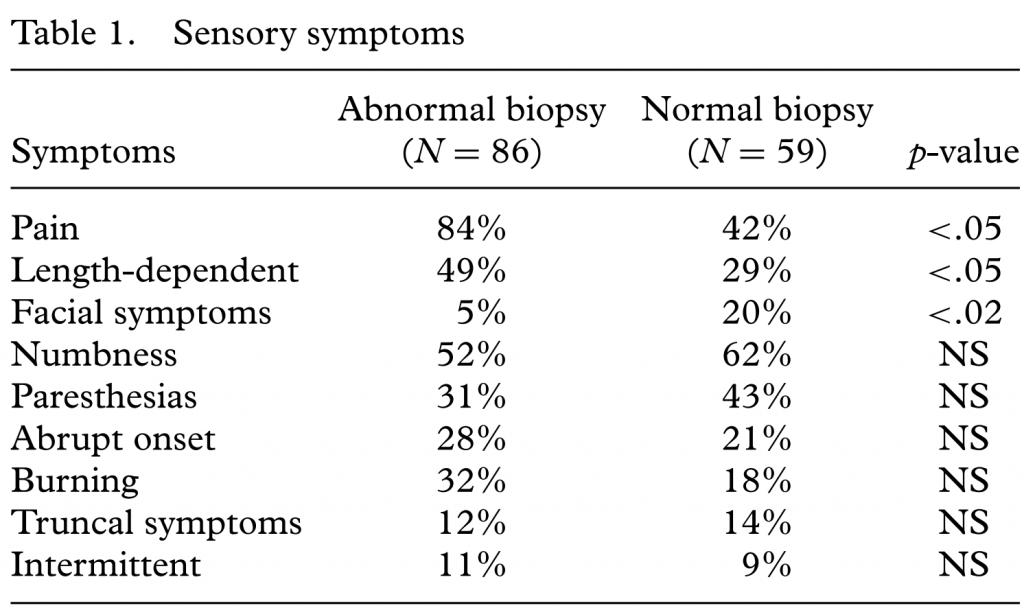
RESULTS
Information from 145 patients was examined. Skin biopsies were taken from both the calf and proximal thigh in 101 patients. Skin biopsy was abnormal (de[1]creased IENFD) in at least one site in 86 patients (59%). There was no significant difference between the two groups with respect to age, gender, or duration of symptoms. Seven patients had abnormal IENFD according to the reference lab’s normal values, but were normal using normal values that were controlled for age and gender. Compared to patients with normal skin biopsies, patients with abnormal skin biopsies were significantly more likely to have pain and a length-dependent pattern of symptoms (Table 1). Otherwise, there were not significant differences between the two groups with respect to sensory symptoms. Approximately 10% of patients had symptoms that were present only intermittently. The two groups showed no significant difference in the frequency of abnormal laboratory test results. A potential etiology was identified in only 44% of patients with presumed SFN (abnormal skin biopsy). These causes were impaired glucose tolerance (17%), diabetes mellitus (8%), vitamin B12 deficiency (7%), and ethanol abuse (4%). Among patients with presumed SFN, a potential etiology was detected almost twice as often in those with a length-dependent pattern compared to patients with a nonlength-dependent pattern (25% vs. 14%). However, this did not reach statistical significance. The response to common neuropathic pain medications (duloxetine, gabapentin, pregabalin, tricyclic antidepressants) differed between patients with and without abnormal skin biopsies. A positive response to any treatment was seen in 84% of patients with an abnormal skin biopsy compared to only 42% of those with a normal biopsy (p < .001). There was no significant difference in the response rate to any specific medication. Among patients with an abnormal biopsy treatment response did not significantly differ between patients with length-dependent and nonlength-dependent patterns.
CONCLUSIONS
The diagnostic yield of skin biopsy was high: 59% of patients with neuropathic symptoms and normal NCS had abnormally reduced by IENFD in at least one biopsy site. This is in line with previous reports studying IENFD in patients with suspected SFN [1, 10, 11]. Clinical symptoms that predicted an abnormal biopsy included pain and length-dependent symptoms. No cause for neuropathy could be determined in 56% of presumed SFN patients. Common identifiable causes included diabetes, impaired glucose tolerance, and B12 deficiency. These findings are also similar to those of other studies [1, 10, 11]. In contrast to the findings of a recent report [5], we did not find any significant difference in age or gender among patients with abnormal skin biopsies showing length-dependent or nonlength-dependent patterns. Most interesting was the observation that an abnormal skin biopsy predicted a high likelihood that a patient would respond to typical neuropathic medications. Among patients with an abnormal skin biopsy, the response rate was 84%—twice the response rate among patients with a normal skin biopsy. Other diagnostic tests may be used to identify SFN. Quantitative sudomotor axon reflex testing (QSART) was found to be abnormal in 68 to 74% of patients with suspected SFN [12, 13]. However, these studies evaluated only patients with distal sensory symptoms. QST is also abnormal in a high percentage of patients with SFN producing distal sensory symptoms. [1, 12] QST, however, is not specific for SFN [1]. QSART and QST, while noninvasive, are not routinely available to most clinicians. In addition, to our knowledge, the ability of QSART or QST to predict patient response to neuro[1]pathic pain therapy has not been evaluated. The cause for sensory symptoms in our patients with normal skin biopsies is uncertain. It is probable some of these patients had a SFN as the diagnostic sensitivity of skin biopsy is not perfect [1,7,8]. However, given the significant difference in treatment response, the majority of the patients with normal biopsies likely had other conditions. Central nervous system or non-neurological processes, or even nonorganic disease, are possible in some. This brings up an important point that supports the utility of performing skin biopsies: a number of our patients with abnormal skin biopsies were initially suspected of having nonorganic disease. This was particularly the case for patients with multifocal sensory symptoms that came and went (a pattern of SFN described by other investigators [1, 14]). Therefore, skin biopsy can be helpful in the diagnosis and management patients with otherwise ill-defined sensory complaints. Finding an abnormal skin biopsy not only increases the likelihood of a treatment response, it also might support more persistent and aggressive attempts at pain control. For example, a number of our patients with presumed SFN had pain refractory to standard neuropathic pain medications, but responded well to chronic opiate therapy. Most clinicians would be more amenable to offering opiates to patients with an objectively confirmed diagnosis of neuropathy than to patients with seemingly vague pain symptoms [15]. In summary, skin biopsy has a relatively high yield in patients with sensory symptoms and no findings of mixed fiber neuropathy on clinical exam or NCS. Ab[1]normal results predict a high likelihood of response to standard neuropathic pain medications. In patients with a symmetrical, length-dependent clinical phenotype of SFN the results skin biopsy may not markedly alter clinical management (although making a definitive diagnosis might benefit patients), but the test may be quite helpful in assessing patients with atypical sensory symptoms. Declaration of interest: The authors alone are responsible for the content and writing of the paper. Drs. Levine and Saperstein have financial interest in a pathology laboratory that processes and analyzes skin biopsies for quantification of IENFD. These interests are not in the laboratory used in this study and were not present at the time this study was performed.
REFERENCES
1. Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, Broglio L, Granieri E, Lauria G. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131:1912–25.
2. Lacomis D. Small-fiber neuropathy. Muscle Nerve. 2002;26:173–188.
3. Gorson KC, Herrmann DN, Thiagarajan R, Brannagan TH, Chin RL, Kinsella LJ, Ropper AH. Non-length dependent small fibre neuropathy/ganglionopathy. J Neurol Neurosurg Psychia[1]try. 2008;79:163–9.
4. Gemignani F, Giovanelli M, Vitetta F, Santilli D, Bellanova MF, Brindani F, Marbini A. Non-length dependent small fiber neuropathy. a prospective case series. J Peripher Nerv Syst. 2010;15:57–62.
5. Khan S, Zhou L. Characterization of non-length-dependent small-fiber sensory neuropathy. Muscle Nerve. 2012;45:86–91. doi: 10.1002/mus.22255.
6. Stewart JD, Low PA, Fealey RD. Distal small fiber neuropathy: results of test of sweating and autonomic cardiovascular reflexes. Muscle Nerve 1992;15:661–5.
7. McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol 1998;55:1513–20.
8. Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, Nolano M, Merkies IS, Polydefkis M, Smith AG, Sommer C, Valls-Sole J. European federation of neurological ´ societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European federation of neurological societies and the peripheral nerve society. J Peripher Nerv Syst. 2010;15:79–92.
9. Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, Smith AG, Hsieh ST, Mellgren SI, Umapathi T, Ziegler D, Faber CG, Merkies IS. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15:202–7.
10. Periquet MI, Novak V, Collins MP, Nagaraja HN, Erdem S, Nash SM, Freimer ML, Sahenk Z, Kissel JT, Mendell JR. Painful sensory neuropathy; prospective evaluation using skin biopsy. Neurology 1999;53:1641–7.
11. Herrmann DN, Ferguson ML, Pannoni V, Barbano RL, Stanton M, Logigian EL. The plantar nerve AP and skin biopsy in sensory neuropathies with normal routine conduction studies. Neurology 2004; 63:879–85.
12. Novak V, Freimer ML, Kissel JT, Sahenk Z, Periquet IM, Nash SM, Collins MP, Mendell JR. Autonomic impairment in painful neuropathy. Neurology. 2001;56:861–8.
13. Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57–61.
14. Wojcicka A, Kinsella LJ. Are fleeting paresthesias due to anxiety or small fiber neuropathy? A reappraisal using skin biopsy. Neurology 2008;70 (Suppl):P01.137.
15. Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D. Evidence-based guideline: Treatment of painful diabetic neuropathy: Report of the American academy of neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–65.
