TODD LEVINE, MD Clinical Associate Professor-University of Arizona, Phoenix Neurological Associates Phoenix, Arizona
DAVID SAPERSTEIN, MD Clinical Associate Professor-University of Arizona, Phoenix Neurological Associates Phoenix, Arizona
The number of patients diagnosed with reflex sympathetic dystrophy (RSD) or complex regional pain syndrome (CRPS) is growing, with current estimates at more than 50,000 new patients per year.1 Yet the diagnosis primarily relies on clinical features (Table 1).
Thus, despite the utility of nerve conduction studies (NCS), triple-phase bone scans, quantitative sensory testing, and medical imaging, many patients are given the diagnosis of RSD/CRPS as one of exclusion. The description of pain, allodynia, and hyperalgesia out of proportion to the objective findings on exam suggest damage to the small unmyelinated nociceptors in the skin. The possibility of a small fiber neuropathy (SFN) should be considered in these patients (Case Study).
Despite the development of standardized tests to assess intra-epidermal nerve fiber density (ENFD), many pain physicians, internists, and neurologists are reluctant to perform these tests. This may be due to unfamiliarity with the process and its easy integration into their practice; or, not being aware of the benefits of objectively determining the cause for the patient’s symptoms. Skin biopsies for ENFD testing on patients with neuropathic pain can be a valuable diagnostic asset, from which many pain physicians and neurologists would most likely benefit.
Nerve Fiber Size: A Brief Review
Nerves are composed of fibers that vary in size and function. The larger, myelinated A-alpha and A-beta fibers convey proprioception and touch. The small fibers consist of the myelinated A-delta and unmyelinated C fibers, which convey pain and temperature. Most peripheral neuropathies affect nerve fibers of all sizes. Such neuropathies are referred to as mixed fiber neuropathies (MFN). In rare cases, only the largest fibers (A-alpha and A-beta) are affected. In these large fiber neuropathies, impaired proprioception is the main deficit. More common than large fiber neuropathies, but less common than MFN, are SFN, wherein only the A-delta and C fibers are involved. Patients with SFN can be difficult to diagnose because they can present with many atypical complaints and a paucity of objective findings on exam.
Patients with SFN typically present with numbness, paresthesias, allodynia, and pain. Pain tends to be a prominent symptom and often has a burning quality. However, there are no unique features of the pain that can, by themselves, distinguish patients with SFN from those with other neuropathies.3 In most cases of SFN, the distribution and character of sensory symptoms and findings will resemble those seen in patients with MFN: symmetrical, length-dependent, and persistent. However, a number of SFN patients will manifest atypical features, such as non–length-dependent and multifocal numbness that may come and go.4-7 These unusual features often can lead to a misconception that the patient’s pain is not organic. This fact highlights the benefits of having a readily available test to objectively prove that an SFN exists in these patients before considering them as functional.
Evidence that the neuropathic symptoms are caused by isolated involvement of the small nerves comes from a neurologic examination revealing preserved deep tendon reflexes, vibratory sensation, and proprioception. Soft touch or pinprick sensation may be reduced slightly but is rarely dramatically affected.
The test that is most useful in diagnosing MFNs— NCS—is normal in SFN. Some patients with SFN also have a concomitant autonomic neuropathy, and therefore autonomic nervous system testing can provide objective confirmation.8,9 Quantitative sudomotor axon reflex testing (QSART) is a useful, noninvasive test. However, access to a facility offering QSART is very limited. Quantitative sensory testing (QST), another noninvasive test, also can support a diagnosis of SFN. However, QST is not entirely objective and, like QSART, is not widely available.
Skin Biopsy for Quantification of Epidermal Nerves
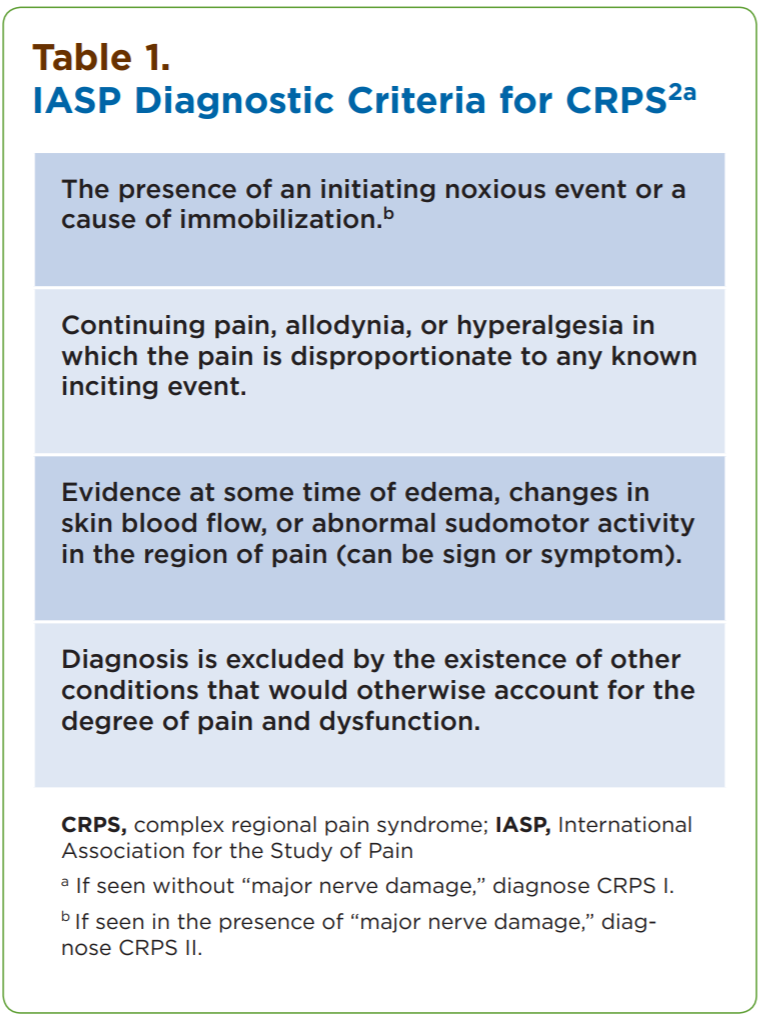
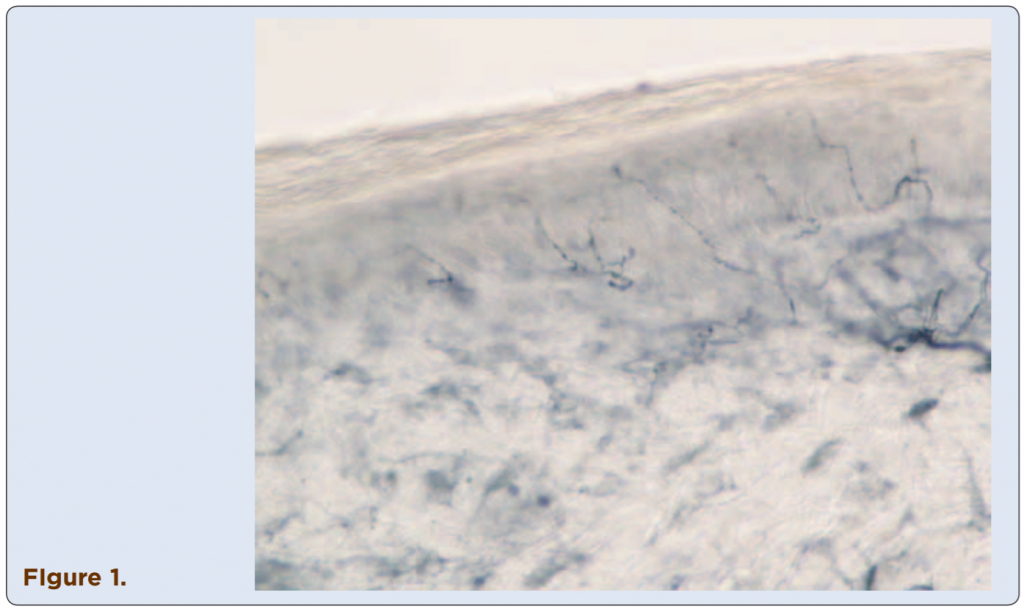
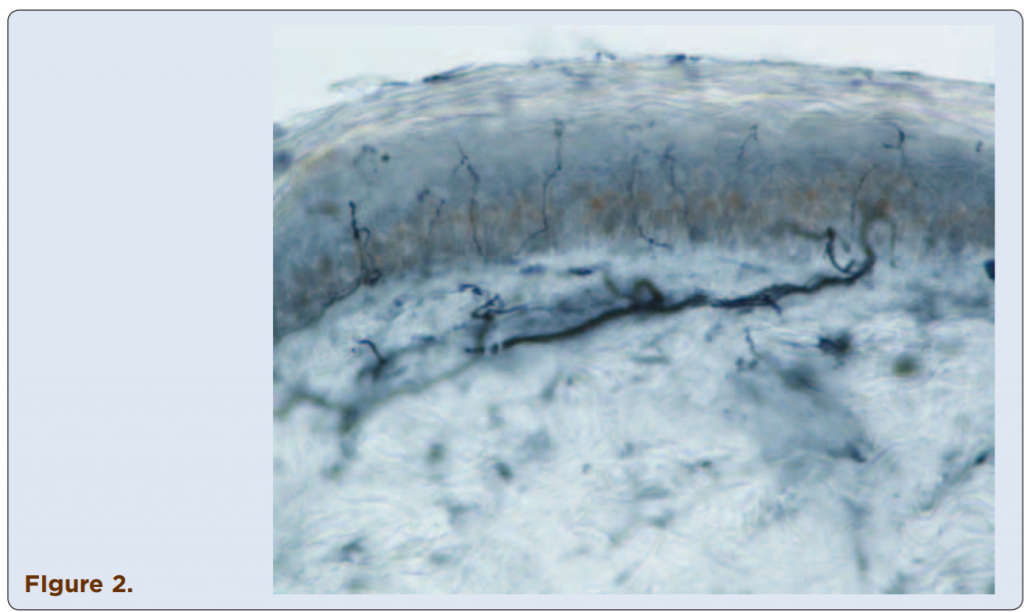
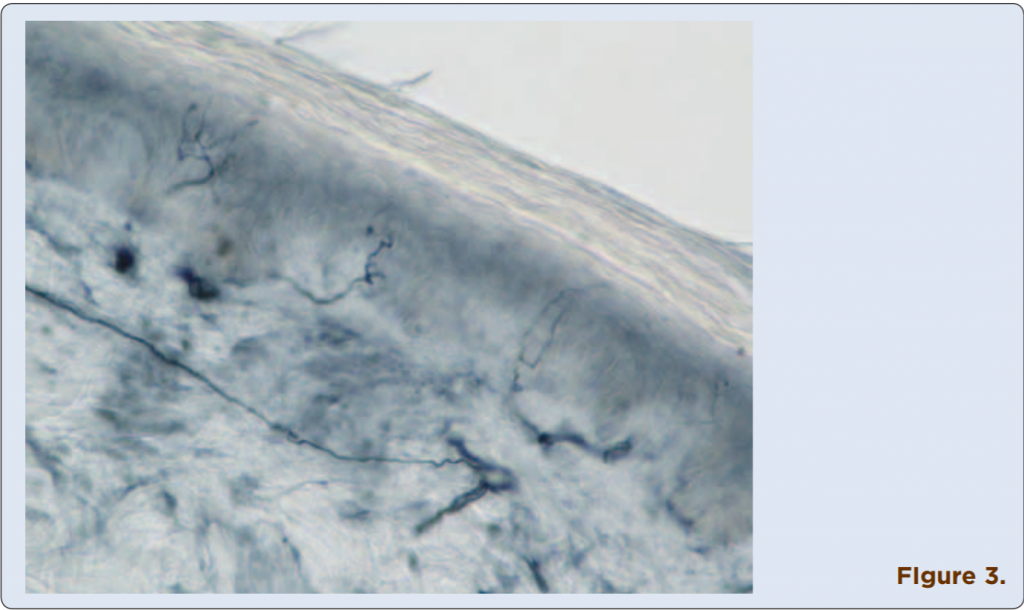
Techniques to identify SFN using skin biopsies have been available for many years at several academic institutions, but this procedure only recently has become commercially assessable to practitioners. Three-millimeter skin punch biopsies are processed for immunohistochemical staining with antibodies against protein gene product (PGP) 9.5, a pan-axonal marker. This allows visualization and quantification of unmyelinated C fibers and possibly myelinated A-delta fibers in the epidermis (Figures 1-4). If the density of epidermal nerve fibers is decreased, as compared with established normative data, then a diagnosis of SFN is supported.
The biopsy technique to obtain the skin samples is easily learned and can be performed quickly. A 3-mm circular punch is used. The specimens are placed in fixative and shipped at room temperature overnight to the commercial lab performing the test. During an elaborate and time-consuming processing procedure, specimens are cut and immunostained with PGP 9.5.10 Individual intra-epidermal nerve fibers can be visualized and are counted manually. These values are compared with established normal values for epidermal nerve fiber count per millimeter available for several sites in legs and arms. Using this technique, the reported sensitivity and specificity is 88% to 92%.10 The use of age- and gender-matched controls may improve specificity,11 but are not used by all labs. The only pain involved is that resulting from injection of lidocaine to numb the small area. The wound can be dressed with a simple bandage and there is minimal scarring. Samples typically are acquired from 2 or 3 standardized sites (such as lower calf, distal thigh, and proximal thigh) or in the areas of most severe pain. It usually is sufficient to obtain biopsies from only a single limb. Having proximal and distal biopsy sites helps determine whether a neuropathy is length-dependent (non– length-dependent findings would support a diagnosis of a ganglionopathy as opposed to a neuropathy, as is seen in Sjögren’s syndrome and some paraneoplastic diseases). Specimens are placed in a vial with fixative and can be sent via overnight shipping to a commercial laboratory. Results are generally available within 2 weeks.
The skin biopsy is a low-risk procedure that any clinician or mid-level practitioner can learn to perform easily. In fact, several commercial laboratories that process the specimens provide video demonstrations, most of which are available online, along with illustrated guides to the biopsy procedure.

The Overlap of Small Fiber Neuropathy and RSD/CRPS
Recently, Oaklander and colleagues proposed that RSD/CRPS may be a post-traumatic neuralgia associated with distal degeneration of the small-diameter peripheral axons.12 They studied 18 adults with IASPdefined CRPS I affecting their arms or legs and examined 3 sites on subjects’ CRPS-affected and matching contralateral limb: the CRPS-affected site, the nearby unaffected ipsilateral control site; and the matching contralateral control site. Seven adults with chronic leg pain, edema, disuse, and prior surgeries from trauma or osteoarthritis provided symptom-matched controls. ENFD was diminished at the CRPS-affected sites of 17 of 18 subjects, on average by 29% (P<0.001).13 ENFD testing provided objective evidence for damage to the small nerve fibers and a clear objective documentation explaining the patient’s pain.>P<0.001).13 ENFD testing provided objective evidence for damage to the small nerve fibers and a clear objective documentation explaining the patient’s pain.
The advantage of being able to objectively document that the patient’s clinical symptoms have a physiologic explanation often is very helpful for the patient psychologically, as well as in cases of personal injury, worker compensation, or disability claims. Additionally, there is reason to expect that patients with objective injury are more likely to respond to typical neuropathic agents. The authors recently reviewed the records of 145 patients in their practice who underwent a skin biopsy for suspected SFN between January 2005 and June 2008. Patients with abnormal NCS or evidence on neurologic exam of a medium or large fiber neuropathy were excluded from the analysis. Patients with an abnormal skin biopsy were more than twice as likely to respond to a first-line neuropathic pain medication, such as amitriptyline, gabapentin (Neurontin, Pfizer), duloxetine (Cymbalta, Lilly), or pregabalin (Lyrica, Pfizer), compared with patients with normal biopsies.7 Patients with small fiber nerve injury may respond differently to invasive procedures such as sympathetic blocks, although controlled trials to address this issue have yet to be performed.
Figures 1-4. Visualized unmylinated C fibers and potentially mylinated A-delta
fibers in the epidermis.
Management of Confirmed SFN
The skin biopsy technique is intended only to confirm a diagnosis of SFN. Unlike sural nerve biopsy, which is usually performed to identify the etiology of neuropathy (such as vasculitis, chronic inflammatory polyneurothapy, or amyloidosis), skin biopsy will seldom disclose an etiology.
In rare cases, skin biopsy may provide evidence for vasculitis or amyloidosis. However, this procedure is not able to “rule out” these possibilities, nor can the skin biopsy prove that the nerve damage is the result of trauma.
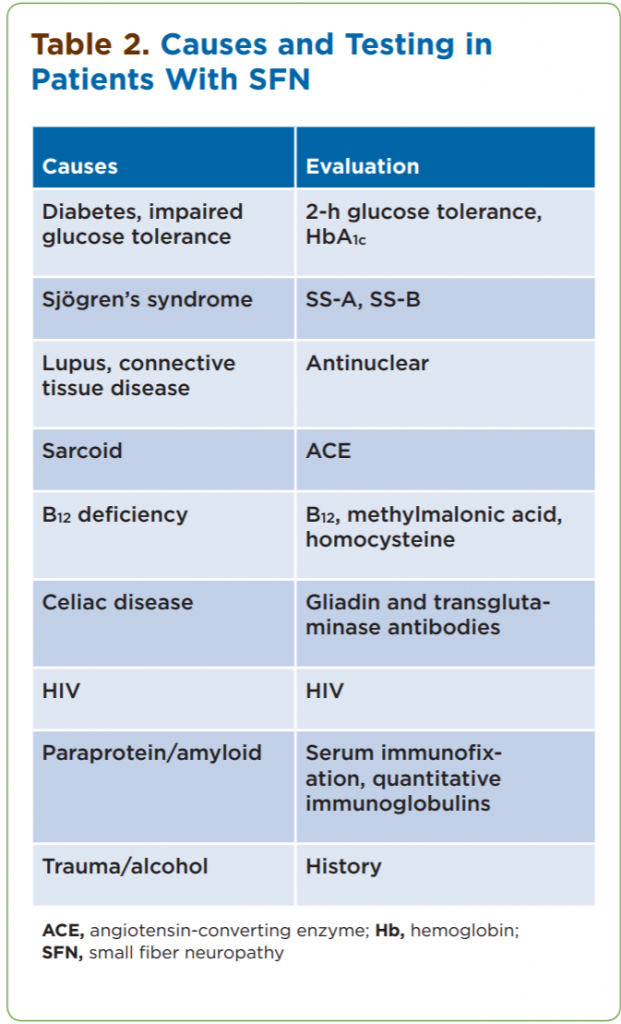
An abnormal skin biopsy should prompt a screen for known causes of SFNs (Table 2).
Despite this extensive evaluation, more than half of SFN cases will be idiopathic.7,8,14,15 The main therapeutic intervention in patients with SFN is symptomatic treatment for neuropathic pain. As noted above, standard first-line therapies (such as duloxetine, gabapentin and pregabalin combination, and gabapentin alone) have proven useful. Additional treatment options include opiates, interventional pain procedures, and even spinal cord stimulators.
Making the Subjective Objective
RSD/CRPS represents a somewhat mixed patient population. Not all of these patients will have damage to their small nerves; however, there are many advantages to being able to objectively demonstrate that a patient has neuropathic injury. It helps clarify the cause for the patient’s pain. An objective diagnosis also may help in treatment choice selections, pointing toward more typical neuropathic pain medications. Being better able to define these patients may help eventually select individuals more likely to respond to interventional pain procedures. The biopsy technique is straightforward, easily learned, widely available, and relatively inexpensive. It is patient-friendly, relatively pain-free, reimbursable, and provides a high diagnostic yield. Because of the ease of obtaining skin biopsies, these tests can be repeated in order to evaluate improvement in response to a given therapy.16 Skin biopsies can easily document progression or improvement. This modality has become widely accepted in clinical trials where changes in ENFD can be seen as early as 3 to 6 months, which is much sooner than one would expect to see changes on NCS.
References
1. de Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHCh, Sturkenboom MCJM. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129(1-2):12-20.
2. Complex Regional Pain Syndromes. International Association for the Study of Pain. In: Core Curriculum for Professional Education in Pain. Charlton JE (ed)., IASP Press, Seattle. 2005.
3. Lacomis D. Small-fiber neuropathy. Muscle Nerve. 2002;26(2):173-188.
4. Gorson KC, Herrmann DN, Thiagarajan R, et al. Non-length dependent small fibre neuropathy/ganglionopathy. J Neurol Neurosurg Psychiatry. 2008;79(2):163-169.
5. Khan S, Zhou L. Characterization of non-length-dependent smallfiber sensory neuropathy. Muscle Nerve. 2012;45(1):86-91. doi: 10.1002/mus.22255.
6. Wojcicka A, Kinsella LJ. Are fleeting paresthesias due to anxiety or small fiber neuropathy? A reappraisal using skin biopsy. Neurology. 2008;70(suppl):P01.137
7. Levine T, Levine M, Hank N, Saperstein DS. Retrospective assessment of the usefulness of skin biopsies in the evaluation and management of patients with suspected small fiber neuropathy. Neurology. 2009;72(suppl 3):A56-A57.
8. Periquet I, Novak V, Collins M, et al. Painful sensory neuropathy; prospective evaluation using skin biopsy. Neurology. 1999;53(8):1641-1647.
9. Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34(1):57-61.
10. Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. J Peripher Nerv Syst. 2010;15(2):79-92.
11. Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15(3):202-207.
12. Oaklander AL, Fields HL. Is reflex sympathetic dystrophy/complex regional pain syndrome type I a small-fiber neuropathy? Ann Neurol. 2009;65(6):629-638.
13. Oaklander AL, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy). Pain. 2006;120(3):235-243.
14. Herrmann DN, Ferguson ML, Pannoni V, et al. The Plantar Nerve AP and Skin Biopsy in sensory neuropathies with normal routine conduction studies. Neurology. 2004;63(5):879-885.
15. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131(Pt 7):1912-1925.
16. Nodera H, Barbano RL, Henderson D, Herrmann DN. Epidermal reinnervation concomitant with symptomatic improvement in a sensory neuropathy. Muscle Nerve. 2003;27(4):507-509.
